
| The Study of Short Lived Species by ESR | 
|
The best way to combine the two interests seemed to me, to do my fourth year research project in magnetic resonance. So I approached Rex Richards, then Professor of Physical Chemistry and Oxford's NMR guru, about the possibility of joining his group. Rex had no vacancies but fortuitously put me in touch with Keith McLauchlan who had just joined the Physical Chemistry department and was starting to build a research group. In conjunction with Peter Atkins, also new to the department, they had planned an ambitious new experiment, to use Electron Spin Resonance (ESR) to investigate the properties of free radicals within a few microseconds of their formation. To set this up they needed a research chemist with a good knowledge of electronics. The post could not have been better suited to my talents and interests and I immediately accepted. After completing my fourth undergraduate year in the group I stayed on for a further three years, researching for my doctorate.
The Experiment
The experiment consisted of producing free radicals in situ
in the cavity of an ESR spectrometer by means of a photolytic flash of light
from a nitrogen-ion laser. Provided the bandwidth of the spectrometer were
wide enough and some high speed data logging were available, it would then be
possible to study the ESR signal as a function of time after its creation.
The basis of the ESR spectrometer was a commercial machine manufactured by Decca Radar. Much of my intial work involved tearing this apart and modifying it to extend its original bandwidth of a few kHz, to around 1MHz.
In order to observe ESR, it is necessary to have a magnetic field and in order to produce the radicals, it is necessary to have a laser. Both of these had been ordered from companies in the USA. Neither arrived on time. Indeed the magnet had not arrived a year after the start of the project and it was necessary to make some hasty non-ESR experiments measuring flame front velocities for oxy-hydrogen explosions, in order to have results for my undergraduate project.
This gave me considerable sympathy for customers' problems, when later in my career I was in the position of a supplier delivering scientific equipment late!
 Results
Results
When sufficient equipment was available to record ESR spectra, a totally unexpected
result was observed.
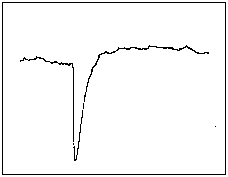
In the first few tens of microseconds of their life, the radicals were found not to be
absorbing RF energy as expected, but to be emitting it. The trace shown
here is the first emission signal ever observed. The total trace length is 4.8mS and
the radicals are formed, by firing a laser pulse 1.2mS after the start of the trace.
The negative spike represents an emission signal and the following positive offset is
the normal absorption signal which decays with a time constant of around 10mS.
We named the process CIDEP (Chemically Induced Dynamic Electron Polarisation). It has proved very fruitfull as a source of information about the reaction process and is still being researched. I derive some quiet satisfaction at being the first person in the world to observe this effect (even though its ultimate understanding and interpretation required the work of others, with more chemical knowledge than I.)
The traces below shows a complete emission spectrum corresponding to a point 100uS after the laser flash. The upper trace represents the result of approximately 2 weeks data gathering of a large number of decay curves which were then laboriously combined to produce a spectrum.
The application of some ingenious electronics allowed the whole process to be automated (an
innovation for its day) and the middle spectrum is the result of some 8 hours data gathering
carried out during the night whilst the author slept!
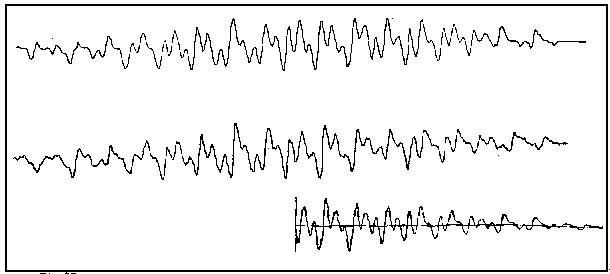
The bottom trace represents a computer simulation of half of the complete spectrum and
provides a means of confirming the radical present. (The diphenyl ketyl radical, obtained from the
photolysis of benzophenone).
It also has a significance as my first foray into the world of computing. It was achieved with the aid of a program written in Algol, punched onto paper tape and fed into the University's English Electric KDF9 computer. If you were lucky, the required output appeared in your pigeon hole the next day. If you were not, all you got was a cryptic error code. A far cry from todays computers but there is no doubt that a 24 hour-per-run turn-around time concentrates the mind to write thoroughly checked bug-free code!